Description
The ART BMC system processes autologous bone marrow aspirate into bone marrow concentrate effectively and efficiently at point-of-care. The system recovers the greatest percentage of nucleated and progenitor cells by allowing selection of the cell concentrate from user-defined portions of the centrifuged stack.
This system is a miniaturized laboratory in a single-use sterile device. The back chamber may be used to store the platelet poor plasma without breaking sterility.
Focused on the safe handling of cells, the ART BMC has multiple ports to accommodate the fewest sterile breaks possible, so that the valuable recovered cells are exposed to the minimum circulating air contaminants.
The built-in back chamber allows for the convenience of adding platelet poor plasma (PPP) back to the bone marrow concentrate (BMC) should more volume be desired for injection; all without additional sterile breaks.
Features
- Functionally closed system minimizes sterile breaks
- You can process anywhere from a minimum of 40 cc up to 60cc aspirate for a variety of applications
- Thumb-wheel allows for selective deconstruction of fluid stack and is designed to accommodate a wide variety of variability of the patient’s hematocrit
- Adjustable flow valve diverts fluid without additional sterile breaks
Benefits
- Efficient concentration yields of ultra-low hematocrit
- Unmatched concentration of molecules, including fibrinogen, alpha-2 macroglobulin, and cytokines of similar molecular weights6
- Customized fluid fractions for tailored final product (e.g. BMC, fibrinogen, A2M, etc.)
- Eliminates turbulence by keeping buffy coat in the collection zone, can yield 3cc to 5cc on average of buffy coat from a typical 60cc bone marrow aspiration
- Consistently achieves high yields of mononuclear cells
Competitive Summary7,8
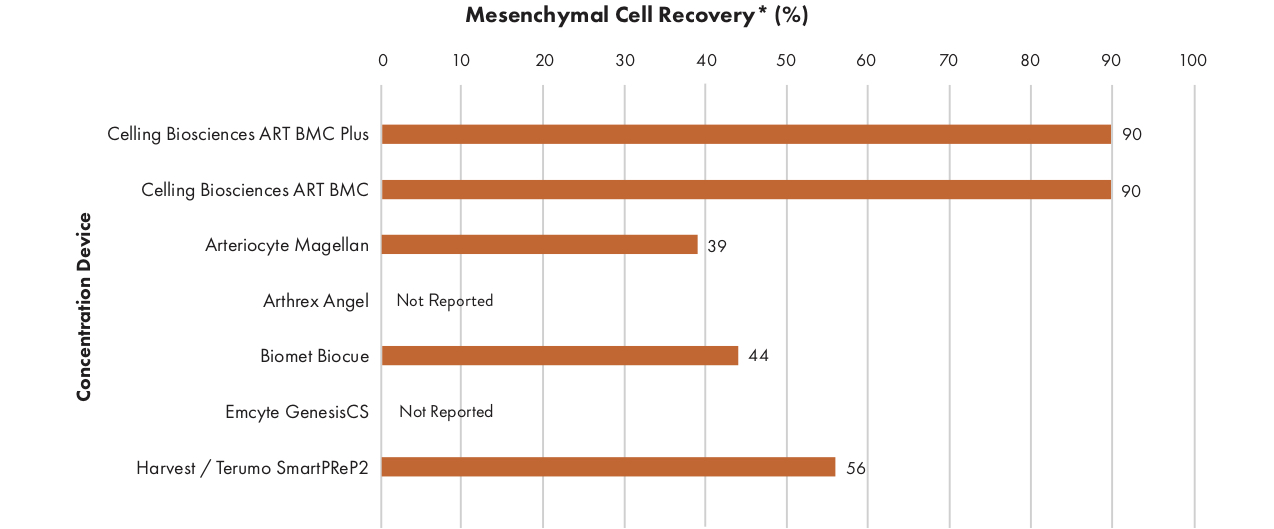 6 Data on File. Celling Biosciences, 2016.
6 Data on File. Celling Biosciences, 2016.
7 Karli et al. “Autologous Regenerative Therapies: Rapid Concentration of Progenitor Cells, Platelets, and Proteins at the Point-of-Care,” TERMIS annual meeting, September 2015, Boston, MA.
8 Hegde et al. “A Prospective Comparison of 3 Approved Systems for Autologous Bone Marrow Concentration Demonstrated Nonequivalency in Progenitor Cell Number and Concentration,” J Ortho Trauma, Vol. 28, October 2014
9 Gassling V. et al. Clin Oral Implant Res 2013 Mar;24(3):320-8
*MSC may be reported as CFU-F or CTP
